Wesley Sundquist, PhD, Samuels Professor and chair of biochemistry at the University of Utah, laid the foundation for the development of a highly effective, long-lasting prophylactic against HIV, which has been named the Breakthrough of the Year by Science, a top scientific journal. The drug lenacapavir, developed by Gilead Sciences, provides protection for half a year instead of one day and has performed extremely well in clinical trials.
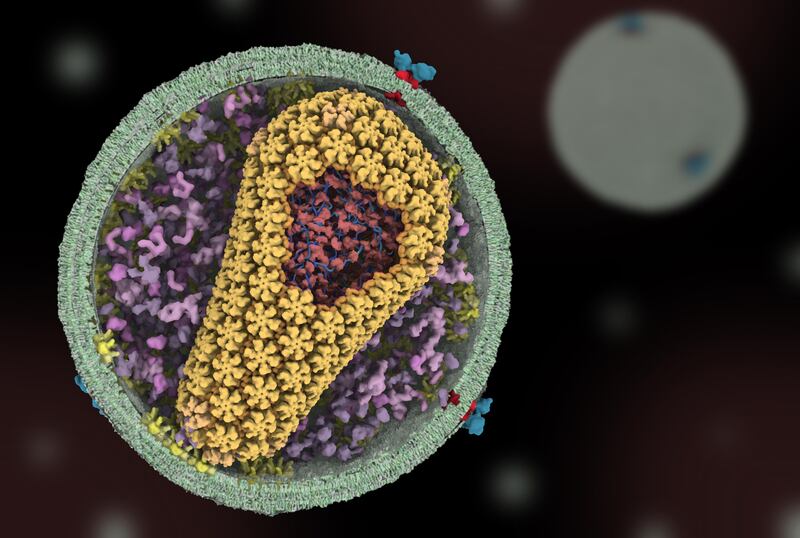
Sundquist’s research focuses on understanding how the HIV virus is built on a molecular level and how it interacts with the body to infect and spread through cells.
By purifying and analyzing the protein shell that surrounds the virus’s genetic material, Sundquist’s team discovered what the shell looks like and how it’s put together. Importantly, the research team found that the virus’s shell is highly sensitive to changes. Making even small tweaks to the proteins that make up the shell stopped the virus from replicating as quickly, which suggested that drugs that affect the protein shell could prove to be effective.
These insights prompted pharmaceutical company Gilead Sciences to search for drugs that target HIV’s protein shell, with Sundquist as a consultant. They ultimately developed lenacapavir, which binds the viral protein shell, preventing it from assembling properly and productively entering the nucleus of host cells. The drug is now used as a second-line treatment for HIV when the virus is already resistant to multiple other drugs.
But lenacapavir’s standout potential is for preventing HIV entirely. “Lenacapavir phase three clinical trials for the prevention of HIV transmission have been spectacularly successful,” Sundquist says. “It’s more potent than any drug available, but more importantly, it’s very long-lasting and effective.”
While other pre-exposure prophylactics (PrEP) against HIV have to be taken every day, a dose of lenacapavir provides protection for six months. Especially in contexts where people have limited access to medical care, the longer duration of lenacapavir leads to a marked difference in outcomes.
In large clinical trials in South Africa and Uganda, not one of the more than 2,000 women who received a dose of lenacapavir contracted HIV over the course of the study. Follow-up trials in other populations, including men and nonbinary people, have confirmed the drug’s efficacy. “Lenacapavir almost completely prevents the transmission of HIV into at-risk populations,” Sundquist sums up. “This is just an amazing result.”
Despite its impacts on human health, Sundquist sees his lab’s work as motivated primarily by discovery. “We’re driven by curiosity to discover things that we don’t understand,” he says. “It’s not so different from other kinds of adventures. The same thing that drives people to climb mountains drives us to discover how molecular machines work.”

